Primary HLH is a rare, genetic immune-mediated disorder characterized by severe immune activation and hyperinflammation. It is associated with progressive immune-mediated organ damage and is fatal if untreated. Once HLH is diagnosed, therapy should be started as soon as possible to reduce the hyperinflammation, then allowing patients to be offered the only potentially curative treatment, namely hematopoietic stem cell transplantation (HSCT). Conventional treatment (CVT) for newly diagnosed patients comprises off-label use of dexamethasone, etoposide and cyclosporine as per the HLH-94 and HLH-2004 protocol (Trottestam et al. Blood 2011; Bergsten et al. Blood 2017). Emapalumab, a fully human monoclonal antibody that neutralizes IFN-gamma, is approved in the USA for treatment of adult and pediatric patients with primary HLH presenting with refractory, recurrent or progressive disease or intolerance to CVT. In the absence of comparators and head-to-head studies, we conducted a naïve, adjusted treatment comparison of emapalumab versus CVT.
A systematic literature review was performed to identify studies that reported clinical efficacy data for CVT in primary HLH. The key relevant data source for CVT (Bergsten 2017), recruited solely treatment-naïve patients. Data from CVT treatment-experienced patients were not identified. Clinical efficacy data for emapalumab were derived from a single-arm trial (Locatelli et al. New Engl J Med 2020) and consisted of treatment-experienced patients who previously received CVT for HLH (mainly re-intensified HLH 94/04 protocol; n=27), aligned to the licensed indication.
A comparison between emapalumab and CVT using simulated trial comparison or match-adjusted indirect comparison was not possible due to scarcity of comparable clinical data and sample size limitations. The comparison of baseline characteristics revealed several differences, which increased uncertainty in the estimates of relative efficacy (Table 1). Two analyses were carried out: an unadjusted comparison between emapalumab and CVT, which was limited due to the inability to account for differences between the two trials, and an adjusted comparison in which a third source was used to correct for the subgroup effect on mortality in a treatment-experienced versus a treatment-naïve population.
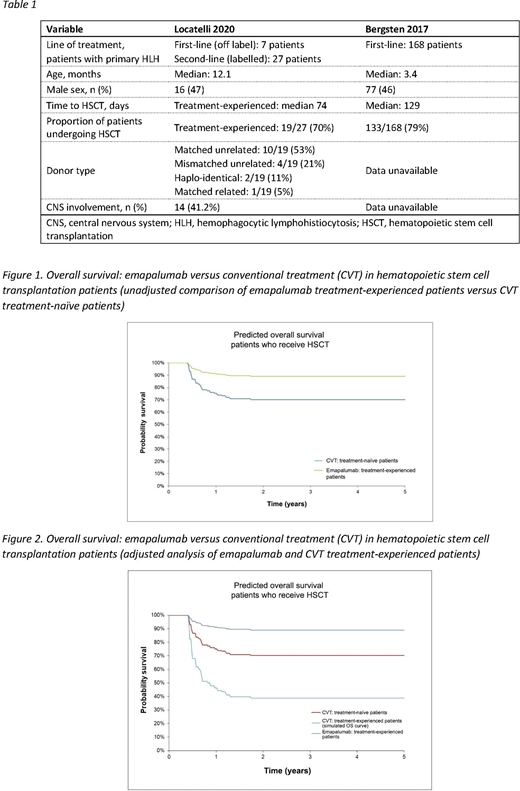
In the unadjusted comparison, post-HSCT overall survival (OS) curves were digitalized from Bergsten 2017. The treatment effect of emapalumab versus CVT was estimated by comparing the mortality observed in treatment-experienced emapalumab patients to the mortality in the CVT-naïve population at 78 weeks using Bergsten 2017 (digitalized 5-year OS curve). The hazard ratio (HR) for mortality was estimated at HR=0.32. The resulting survival trends are captured in Figure 1. It was considered that treatment-experienced patients are expected to be in a poorer condition and have worse prognosis than treatment-naive patients; therefore, the unadjusted comparison may be considered as conservative for emapalumab.
For the adjusted comparison, the data from Mahlaoui et al. Pediatrics 2007 was used to account for the subgroup effect (that treatment-experienced patients have worse prognosis than treatment-naïve patients). In this study, 17/22 (77%) treatment-naive and 4/8 (50%) treatment-experienced patients survived to the end of the study. The HR for treatment-naive versus treatment-experienced patients was estimated to be HR=2.69. The HR for this subgroup was used to adjust survival outcomes reported for the treatment-naive population in Bergsten 2017 (HR=0.32), in order to predict survival for a CVT-experienced population (i.e. generate an estimated survival curve assuming patients were treated with re-intensified HLH-2004). The subgroup effect was assumed to decrease from HR=2.69 at week 78 to HR=1.0 at 2 years, which implied that no further subgroup effect has been assumed beyond 2 years. The resulting OS trends are reported in Figure 2.
OS estimates were consistent and favored emapalumab over frontline CVT both in the unadjusted and adjusted comparisons, however, there were limited data to inform the adjusted comparison; the only available study, Mahlaoui 2007, had a small sample size. Emapalumab may provide an efficacy benefit compared with CVT for the treatment of primary HLH patients who are intolerant or refractory to CVT.
Dewilde:Sobi: Consultancy. Nivelle:Sobi: Consultancy. Griffiths:Sobi: Consultancy. Wilson:Sobi: Current Employment, Current equity holder in publicly-traded company. Nazir:Sobi: Current Employment. Pochopien:Sobi: Consultancy. Locatelli:Jazz Pharmaceeutical: Speakers Bureau; Medac: Speakers Bureau; Miltenyi: Speakers Bureau; Bellicum Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal